Introduction:
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare nonmalignant clonal disorder of hematopoietic stem cells characterized by a phosphatidylinositol glycan protein A (PIGA) gene mutation. This mutation results in the deficiency of glycosylphosphatidylinositol (GPI)-anchored proteins, CD55 and CD59, on the cell surface, rendering red blood cells (RBC) susceptible to complement-mediated destruction. This leads to hemolytic anemia, thromboses, and bone marrow failure. Here we describe two cases of PNH presenting to a tertiary care center.
Case 1
A 29-year-old male with a history of polysubstance use presented to the Emergency Department with a 2-day history of progressively worsening left lower quadrant abdominal pain. CT abdomen pelvis showed multiple renal infarcts and findings suggestive of pneumatosis intestinalis. A complete blood count (CBC) revealed pancytopenia with a leukocyte count (WBC) of 3 x 10 9/L, hemoglobin (Hb) of 94 g/L, and platelet count (Plt) of 48 x 10 9/L. Lactate dehydrogenase (LDH) was elevated (900 IU/L), haptoglobin level was low ( <8mg/dL), and direct antiglobulin test (DAT) was negative. Urine was positive for hemosiderin, and flow cytometry showed RBCs (6.7%), granulocytes (97.97%), and monocytes (89.02%) with PNH phenotype. Bone marrow biopsy (BMB) was negative for dysplasia and fibrosis with normal hematopoiesis but showed increased erythroid precursors. Enoxaparin was started for thromboses and levofloxacin for neutropenia. Treatment for PNH was initiated with a loading dose of ravulizumab and meningococcal vaccination. The patient was lost to follow-up after receiving the first maintenance dose.
Case 2
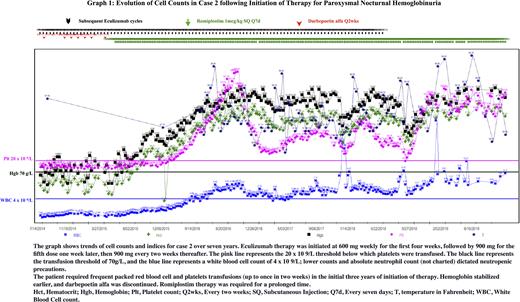
An 81-year-old female with a medical history of hypertension and coronary artery disease was hospitalized for gradually worsening shortness of breath and fatigue ongoing for 2 months. She reported easy bruising but denied a history of obvious bleeding from any orifice. Physical exam was significant for petechial rash in her lower extremities. Labs revealed pancytopenia (WBC 1.44 x 10 9/L, Hb 68 g/L, Plt 8 x 10 9/L), low reticulocyte count, LDH of 276 IU/L, and negative DAT. BMB showed marked aplasia, and she became transfusion dependent. Subsequently, flow cytometry revealed PNH phenotype in 1.45% of RBCs, 54% granulocytes, and 54% monocytes. Eculizumab therapy was started for the diagnosis of PNH. However, her transfusion requirements did not decrease. She developed iron overload and showed positive conversion on DAT. Darbepoetin alfa and romiplostim were added. Her Hb stabilized at >70g/L in 3 years, following which she only required romiplostim to maintain Plt >20,000 ( Graph 1). After 5 years, she opted to discontinue all treatment. She is being followed up regularly and has since remained stable.
Discussion
The two cases above are reflective of how PNH can present in different ways, ranging from the classic triad of symptoms to those with subclinical findings associated with other underlying conditions, such as aplastic anemia or myelodysplastic syndrome. The introduction of complement inhibitors completely changed the natural history of PNH, as patients' median survival shifted from 15-20 years to align with age-matched controls.
Indications for treatment include severe anemia, thrombosis, or PNH symptoms like dyspnea, fatigue, and painful paroxysms. Currently, eculizumab and ravulizumab are the only FDA-approved drugs for PNH treatment. Ravulizumab is non-inferior to eculizumab, with the added advantage of less frequent dosing (every 8 weeks versus 2 weeks).
Regular monitoring for hemolysis is warranted with treatment. Many patients require long-term therapy, which continues to be a significant challenge, even with the spaced dosing of ravulizumab.
Eculizumab protects from terminal complement-mediated damage preventing intravascular hemolysis. However, these cells are still deficient in CD55, making them vulnerable to opsonization by C3 and premature removal in the spleen. As a result, >50% of PNH patients receiving treatment show positive conversion on DAT, as seen in case 2.
Heparin is preferred in acute thrombotic states. Most hematologists recommend anticoagulation till a few months after starting complement inhibition.
Disclosures
No relevant conflicts of interest to declare.